Archive for September 2022

An academic’s guide to medtech translation
This online guide is designed for academics and researchers and will be useful for anyone with an interest in medical technologies innovation and translation, with a focus for innovators based in higher education or research institutions.
Read More
Bridging the innovation funding gap: Maximising proof of concept success
This free good-practice guide for knowledge exchange and commercialisation professionals introduces a new, holistic approach to proof of concept schemes for any sector.
Read More
Back to academic roots after industry success
Dr Helen Berry’s career journey has come full circle, from academia to industry and now back again.
Read More
Enamel repair technology trialled on human teeth
A method of repairing tooth enamel, developed with IKC support and additional funding from the EU, Innovate UK and EPSRC, is being trialled on human teeth.
Read More
How a ‘smart implant’ could help joint replacement patients with recovery
A new device able to reliably monitor a patient’s recovery following joint replacement will soon be ready for regulatory approval.
Read More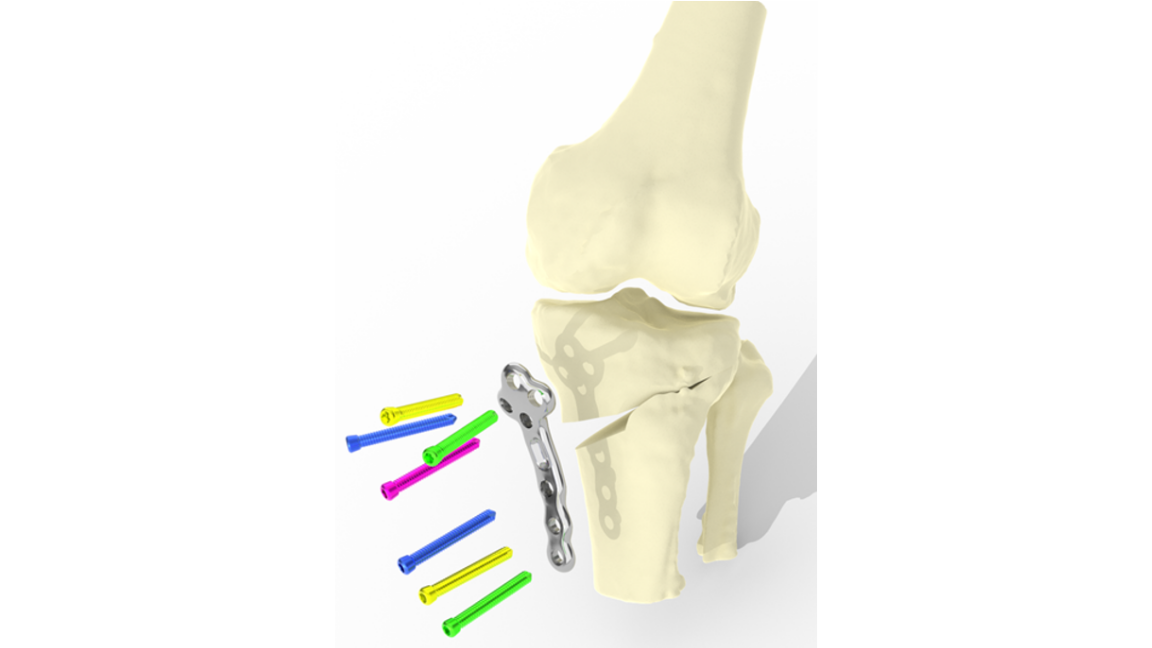
Trial success for personalised knee surgery
Technology that enables complex knee surgery to be personalised for each individual has been successfully trialled in patients for the first time. The ‘TOKA surgical system’ – supported by the IKC during its early development – is set to enter a larger UK trial later this year.
Read More
Bone and cartilage scaffold to enter clinical trials
An osteochondral scaffold that can repair large cartilage defects is due to go into its first clinical trials later this year.
Read More
Infection control in gum disease
A technology to tackle infection and inflammation in serious gum disease and enable the tissue to regenerate has shown promise in laboratory tests.
Read More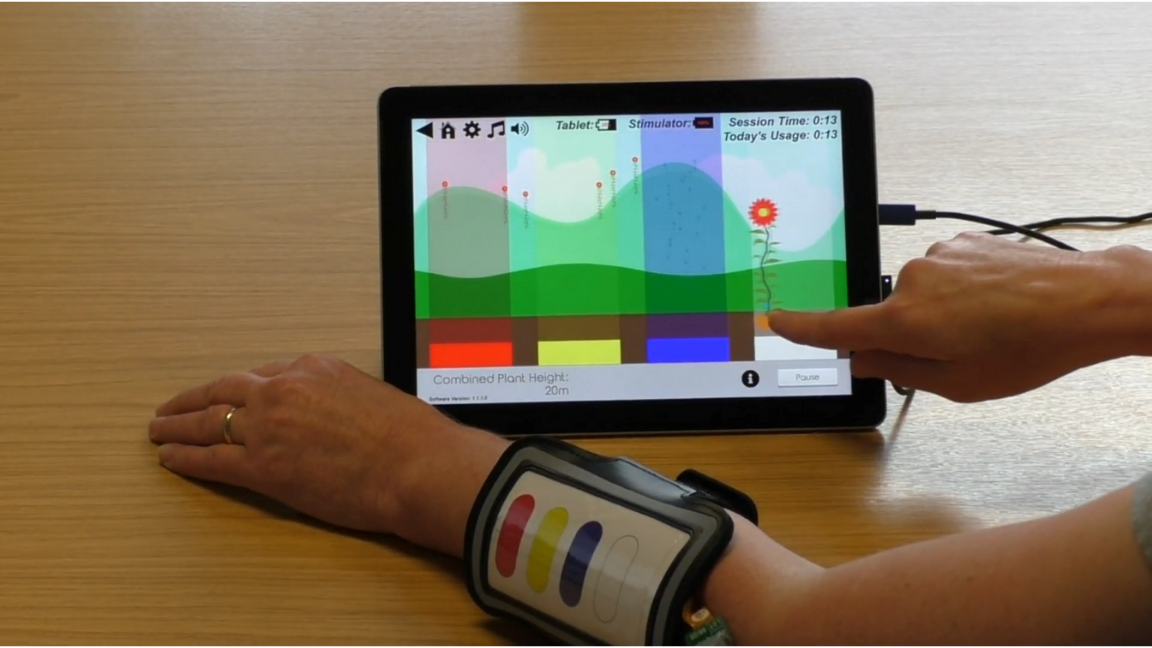
‘Gaming’ device aims to help with persistent pain
An innovative device to tackle chronic limb pain is being trialled across the UK, thanks to support from the IKC and Versus Arthritis.
Read More
Boosting career progression: from academia to industry
Dr Tom Baboolal’s career transition from academic research in cell biology to a clinical researcher position in industry was made possible through the IKC.
Read More
